A) I,II,and III
B) II,IIIand IV
C) IIIand IV
D) I,IIand IV
E) III,IVand V
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Henderson-Hasselbach equation,used to calculate the pH of simple conjugate-pair buffer systems,would be expressed for an ammonia/ammonium chloride buffer,for which Kb(NH3) is 1.8 × 10-5,as:
A) pH = 4.74 + log([NH3]/[NH4+])
B) pH = 4.74 + log([NH4+]/[NH3])
C) pH = 9.25 + log([NH3]/[NH4+])
D) pH = 9.25 + log([NH4+]/[NH3])
E) pH = 14.0 - log(1.8 × 10-5)
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 30.0 mmol HCl(g) is added to 1.00 L of a buffer that is 0.340 M NH3(aq) and 0.290 M NH4Cl(aq) ,what are the final concentrations of NH3(aq) and NH4Cl(aq) ,respectively? Assume no volume change.
A) 0.310 M and 0.320 M
B) 0.310 M and 0.290 M
C) 0.370 M and 0.290 M
D) 0.370 M and 0.320 M
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An aqueous solution is prepared by dissolving 0.23 mol of nitrous acid and 0.27 mol of sodium nitrite in water sufficient to yield 1.00 L of solution.The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly.The pH does not decrease drastically because the HCl reacts with the ________ present in the buffer solution.The 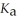 Of nitrous acid is 1.36 × 10-3.
Of nitrous acid is 1.36 × 10-3.
A) ![]() O
O
B) ![]()
C) nitrite ion
D) nitrous acid
E) This is a buffer solution: the pH does not change at all upon addition of acid or base.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What will the pH at the neutralization point of 0.00812 M Ba(OH) 2(aq) be when titrated with HCl(aq) ?
A) 7.0
B) 12.2
C) 8.0
D) 9.0
E) 6.0
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
25 mL of 0.10 M aqueous acetic acid is titrated with 0.10 M NaOH(aq) .What is the pH after 30 mL of NaOH have been added? Ka for acetic acid = 1.8 × 10-5.
A) 12.0
B) 2.0
C) 12.2
D) 8.7
E) 12.3
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the pH of the following aqueous solution.Initial concentrations are given. [HF] = 1.296 M,[NaF] = 1.045 M,Ka for HF is 6.6 × 10-4
A) 10.73
B) 3.27
C) 3.18
D) 3.09
E) 11.91
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An aqueous solution containing equimolar amounts of a weak acid with Ka = 10-5 and its sodium salt has:
A) pH > 7
B) pH < 7
C) pH = 7
D) pH dependent on concentration ratios
E) pH dependent on the nature of the acid anion
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a buffer that is 0.88 M HCN(aq) and 0.53 M NaCN(aq) ? The Ka of HCN is 6.2 × 10-10.
A) 8.99
B) 9.21
C) 9.43
D) 4.79
F) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Acid-base indicators have two forms: an acid of one color and a base of another color.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following titration,determine whether the solution at the equivalence point is acidic,basic or neutral and why: HCl(aq) is titrated with NH3(aq)
A) acidic because of hydrolysis of NH4+
B) basic because of hydrolysis of NH3
C) acidic because of hydrolysis of Cl-
D) acidic because of hydrolysis of HCl
E) neutral salt of strong acid and strong base
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Twenty-five milliliters of 0.10 M HCl(aq) is titrated with 0.10 M NaOH(aq) .What is the pH before any NaOH(aq) is added?
A) 0.40
B) 2.5
C) 0.1
D) 1.0
E) 25
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For HClO2,Ka = 1.2 × 10-2.What is the pH of an aqueous solution in which [NaClO2] = 0.193 M and [HClO2] = 0.203 M?
A) 3.32
B) 0.11
C) 1.92
D) 1.94
E) 1.90
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How will addition of sodium acetate to an aqueous acetic acid solution affect the pH?
A) It will lower the pH.
B) The pH will not change.
C) The solution becomes hotter.
D) The pH cannot be measured.
E) It will raise the pH.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the percent ionization of nitrous acid in an aqueous solution that is 0.266 mol L-1 in nitrous acid 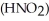 And 0.112 mol L-1 in potassium nitrite (
And 0.112 mol L-1 in potassium nitrite ( 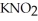 ) .The acid dissociation constant of nitrous acid is
) .The acid dissociation constant of nitrous acid is 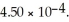
A) 29.6
B) 0.402
C) 11.2
D) 1.34 × ![]()
E) 1.55
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following titration,determine whether the solution at the equivalence point is acidic,basic or neutral and why: KOH(aq) is titrated with HI(aq)
A) basic because of hydrolysis of K+
B) basic because of hydrolysis of KOH
C) acidic because of hydrolysis of OH-
D) acidic because of hydrolysis of HI
E) neutral salt of strong acid and strong base
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Formic acid (HCOOH,Ka = 1.8 × 10-4) is the principal component in the venom of stinging ants.What is the molarity of a formic acid solution if 25.00 mL of the aqueous formic acid solution requires 29.80 mL of 0.0567 mol L-1 NaOH(aq) to reach the equivalence point?
A) 0.0134 mol L-1
B) 0.0476 mol L-1
C) 0.0567 mol L-1
D) 0.0676 mol L-1
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A buffer was prepared by adding 2.4 g of ammonium nitrate to 100.0 mL of 0.30 M aqueous ammonia (Kb = 1.8 × 10-5) .To this solution was then added 10.0 mL of 0.30 M aqueous sodium hydroxide,which caused a pH change of ________.
A) 3.0 pH units
B) 0.30 pH units
C) 0.90 pH units
D) 0.09 pH units
E) zero
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The buffer capacity of a solution may be defined as the number of moles of H+ that will change the pH of 1.00 L of the buffer by 1.00 pH units.What is the buffer capacity of an aqueous solution which is 0.10 M in acetic acid (Ka = 1.8 × 10-5) and 0.30 M in sodium acetate,in units of mol (H+) per liter?
A) 0.20
B) 0.10
C) 0.087
D) 0.009
E) zero
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hydronium ion concentration in an aqueous solution prepared by mixing 50.00 mL of 0.10 mol L-1 HCN with 50.00 mL of 0.010 mol L-1 NaCN? Assume that the volumes of the solutions are additive and that Ka = 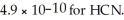
A) 4.9 × 10-11 mol L-1
B) 4.9 × 10-10 mol L-1
C) 4.9 × 10-9 mol L-1
D) 7.0 × 10-6 mol L-1
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 130
Related Exams